This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop first effective preclinical models for most common genetic cause of Leigh syndrome

Researchers from Children's Hospital of Philadelphia (CHOP) developed two new zebrafish models for studying a specific genetic form of mitochondrial disease that represents the most common cause of Leigh syndrome. Using these models, the team identified two drugs already approved by the Food and Drug Administration (FDA) for other conditions that could be repurposed to treat this specific cause of Leigh syndrome. The findings were recently published in the journal Human Molecular Genetics.
SURF1 deficiency is the most common nuclear genetic cause of Leigh syndrome. The SURF1 gene is critical for transforming nutrients into energy that can be used by our cells. Disease-causing mutations in the SURF1 gene disrupt mitochondrial respiratory chain complex IV activity, preventing energy from being made and often leading to complex medical problems.
The specific pattern of disease most commonly seen in affected patients is Leigh syndrome, which frequently involves metabolic strokes and seizures, developmental delays, peripheral neuropathy, and in some cases, early childhood mortality. No FDA-approved therapies currently exist for treating Leigh syndrome or any disease caused by issues related to SURF1.
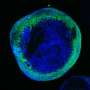
Though several other preclinical models have been developed to study SURF1 disease, many of those attempts either did not accurately replicate the disease progression experienced by patients or did not readily respond to study potential therapeutic interventions. To address this issue, the researchers at the Mitochondrial Medicine Program at CHOP used CRISPR technology to create and characterize two novel zebrafish models that more accurately represent the clinical issues encountered in patients living with SURF1 deficiency.
"Creating preclinical models that reproduce major characteristics of individual genetic causes of complex rare diseases are critical to enable researchers teams to better understand the mechanisms underlying health problems and to discover potentially effective treatments for diverse aspects of multi-system disorders such as Leigh syndrome, which may negatively impact patients through a variety of life-challenging symptoms," said Marni Falk, MD, Professor of Pediatrics and Executive Director of the Mitochondrial Medicine Program at CHOP and senior co-author of the study.
"We have had tremendous success applying the newest genetic technologies to establish and characterize zebrafish preclinical models for a variety of mitochondrial diseases, and this latest study demonstrates how important disease-specific models are for us to identify potential therapies specific to major disease subsets of patients who currently have limited treatment options."
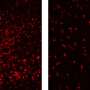
The researchers found that as SURF1-deficient zebrafish aged, they developed structural problems with their eyes and decreased swimming activity, the latter a sign of diminished energy levels and a hallmark feature of mitochondrial diseases. These zebrafish larvae also demonstrated acute stress sensitivity, another critical hallmark of Leigh syndrome that can induce developmental regression and neurologic degeneration due to metabolic strokes.
However, when these zebrafish were treated with either cysteamine bitartrate or N-acetylcysteine, their oxidative stress defenses improved, which the researchers evaluated by measuring increased levels of glutathione, a substance involved in tissue repair. Furthermore, these treatments made the zebrafish much more resisant to stressor-induced brain death, swimming and neuromuscular dysfunction, and loss of heartbeat.
"These studies suggest that this therapeutic class of treatments may hold potential benefit to prevent neurologic decompensation in human patients with SURF1, or potentially other genetic forms of Leigh syndrome," said study co-first author Suraiya Haroon, Ph.D., research assistant professor in the the Mitochondrial Medicine Program at CHOP.
An international, multi-site natural history study for all known causes of Leigh syndrome, including SURF1 disease, is currently underway to better certain outcome measures that may be used to study new therapies in future treatment trials.
"Rigorous clinical trials will be needed to objectively determine whether these drugs can prevent neurodevelopmental decompensation and/or improve neurologic outcomes in mitochondrial disease patients with Leigh syndrome spectrum disorders," Falk said.
"However, these translational research findings represent such an important leap forward in our understanding of the importance of oxidative stress in this particular form of mitochondrial disease and providing objective evidence that boosting oxidative stress defenses may yield meaningful clinical benefit."
More information: Suraiya Haroon et al, N-Acetylcysteine and cysteamine bitartrate prevent azide-induced neuromuscular decompensation by restoring glutathione balance in two novel surf1 −/− zebrafish deletion models of Leigh syndrome, Human Molecular Genetics (2023). DOI: 10.1093/hmg/ddad031